News
Fujifilm wins FDA nod for AI-powered endoscopy tech

Google News Recentlyheard
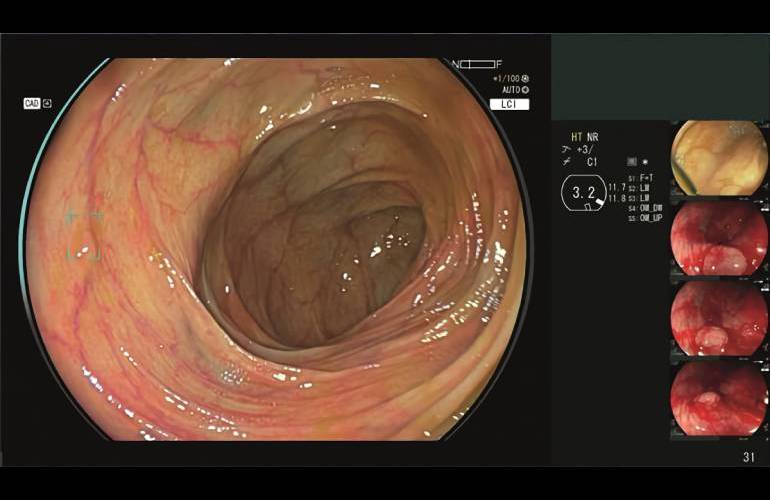
Fujifilm launched proper now that the FDA granted 510(okay) clearance for its CAD Eye AI-powered detection system for endoscopic imaging.
CAD Eye permits the real-time detection of colonic mucosal lesions like polyps and adenomas all through colonoscopy procedures. It helps endoscopists in detecting and eradicating pre-cancerous lesions, regardless of measurement, kind and color. The system enters a market at current dominated by Medtronic and its GI Genius system.
GI Genius, a computer-aided polyp detection system powered by AI, first grew to change into on the market throughout the U.S. in 2021. Be taught further about Medtronic’s AI-related efforts for GI Genius proper right here.
Every devices have been designed to help endoscopists in detecting colonic mucosal lesions. CAD Eye works with every white gentle imaging and Linked Color Imaging (LCI). Fujifilm included an enhanced visualization mode to tell apart the pink color spectrum, enhancing mucosal visualization. Anthony Borrelli, govt director of endoscopy product & promoting at Fujifilm Healthcare Americas Firm suggested MassDevice that the LCI capabilities, together with HD white gentle, are a key differentiator with regard to the aggressive GI Genius.
Fujifilm’s system incorporates an acceptable enlargement unit (the Fujifilm EX-1) and endoscopy help software program program (EW10-EC02). CAD Eye builds upon the company’s Eluxeo endoscopic imaging system, with AI image processing for integration with the system’s processor and endoscope.
The company plans to make CAD Eye commercially on the market this spring after it completes a restricted market evaluation.
“Each single day, we delight ourselves on delivering what we think about is the most effective top quality imaging and optics, arming endoscopists with the devices they need to struggle this public nicely being downside,” acknowledged Tai Fujita, GM, endoscopy, Fujifilm Healthcare Americas Firm. “At current, we’re thrilled to take {{that a}} step extra with the introduction of CAD Eye, which has the potential to dramatically improve the usual of colonoscopy.”
Totally different potential differentiators
In step with Borelli, Fujifilm’s CAD Eye system’s inside progress and AI teaching moreover separate the experience from the pack. Builders educated the system 100% on photos captured by Fujifilm endoscopy imaging instruments.
Borelli acknowledged: “Fujifilm believes this to be a great energy as the company is known for its fantastic optics and movie top quality – significantly in its endoscopic imaging applications – which we think about strengthens the CAD Eye product.”
Furthermore, it affords seamless integration proper into a health care provider’s workflow. Physicians can activate and deactivate CAD Eye on the endoscopic take care of, with out having to point out in the direction of the instruments tower, take their eyes off the affected particular person or ask for help.
“Purchaser ideas signifies that may be very impactful for his or her workflows and a strong differentiator,” Borelli added.
Further regarding the Fujifilm CAD Eye system
The company developed CAD Eye using deep finding out experience in its Tokyo-based worldwide AI experience coronary heart. Fujifilm validated the system using histologically confirmed polyps in scientific photos.
In step with a data launch, the experience helps the detection of lesions that may present easy to miss. This accommodates flat lesions, these on the nook of the endoscopic view and numerous lesions present in a single physique. When CAD Eye identifies a suspicious polyp, the physician mechanically receives every seen and auditory alerts. Seen cues overlay, nevertheless don’t intrude with the scientific photos inside the current workflow.
Fujifilm acknowledged analysis demonstrated CAD Eye’s talent to end in considerable advances in colorectal most cancers detection and evaluation. The system detects significantly further adenomas all through screening and surveillance as compared with commonplace high-definition colonoscopy with out AI assist. Plus, that occurs with none enhance in course of time.
Furthermore, Fujifilm observed 17% better adenoma per colonoscopy when as compared with high-definition commonplace colonoscopy. The system detects colorectal neoplastic lesions at a level akin to that of an skilled and superior to that of a beginner, the company acknowledged.
“As a gastroenterologist dedicated to affected particular person care and safety, I’m heartened by the FDA’s approval of Fujifilm’s AI CAD polyp detection algorithm. This breakthrough not solely permits the early detection of precancerous lesions which will lead as a lot as colorectal most cancers however moreover significantly reduces the hazard of missed lesions, enhancing our precision and bettering affected particular person outcomes,” acknowledged DR. Sravanthi Parasa of the Swedish Medical Coronary heart. “It marks a pivotal improvement in our talent to safeguard affected particular person nicely being, reinforcing our dedication to embracing technological enhancements in gastrointestinal healthcare.”
-

 Business4 weeks ago
Business4 weeks agoProcess Modeling Tools in Business Analysis
-
 Education4 weeks ago
Education4 weeks agoTop 6 AI Writing Tools for Everyday Use
-
Health4 weeks ago
Understanding Spinal Stenosis and How It’s Treated
-

 Finance3 weeks ago
Finance3 weeks agoNavigating the Complex World of Student Loans: Tips for Borrowers
-
 Technology2 weeks ago
Technology2 weeks agoHow to Unlock Your iPhone if Forgot Passcodes?
-

 News1 week ago
News1 week agoKim Mulkey Attacks Potential Washington Post Story Before Its Release
-

 News1 week ago
News1 week agoTammy Murphy suspends campaign for New Jersey US Senate seat
-

 News1 week ago
News1 week agoChick-Fil-A backtracks from its no-antibiotics-in-chicken pledge











